The U.S. Government and Antimicrobial Resistance
Nellie Bristol. "The U.S. Government and Antimicrobial Resistance." CSIS Commission on Strengthening America's Health Security, Center for Strategic and International Studies, June 09, 2020. Accessed October 01, 2025. https://healthsecurity.csis.org/articles/the-u-s-government-and-antimicrobial-resistance/
THE ISSUE
Infection-causing pathogens, including bacteria and fungi, can develop the ability to survive the drugs intended to kill them, a phenomenon known as antimicrobial resistance (AMR). Loss of antimicrobial effectiveness means illnesses that previously could be treated quickly and easily, including food-borne ailments, pneumonia, and health care associated-infections, can instead turn into costly, long-term medical ordeals that may ultimately prove lethal. It is a slow-moving epidemic that will take full advantage of the dislocations and new medical needs generated by the coronavirus pandemic. Already in the United States, resistant infections afflict more than 2.8 million people a year, resulting in at least 35,000 deaths annually. In addition to causing direct morbidity and mortality, AMR threatens medical advances in cancer, organ transplants, burns, and even joint replacement, all of which depend on antibiotics for effective treatment. The coronavirus pandemic adds additional urgency to the issue, as thousands of patients will develop dangerous secondary infections that could prove deadly. Addressing AMR requires rigorous prevention and antibiotic stewardship strategies and a steady pipeline of effective therapies. While the United States and Europe are responding to this growing global threat, scaled-up mitigation strategies and dependable prevention and antibiotic development financing are urgently needed throughout the world.
IMMEDIATE U.S. ACTION REQUIRED TO COMBAT DANGEROUS INFECTIONS
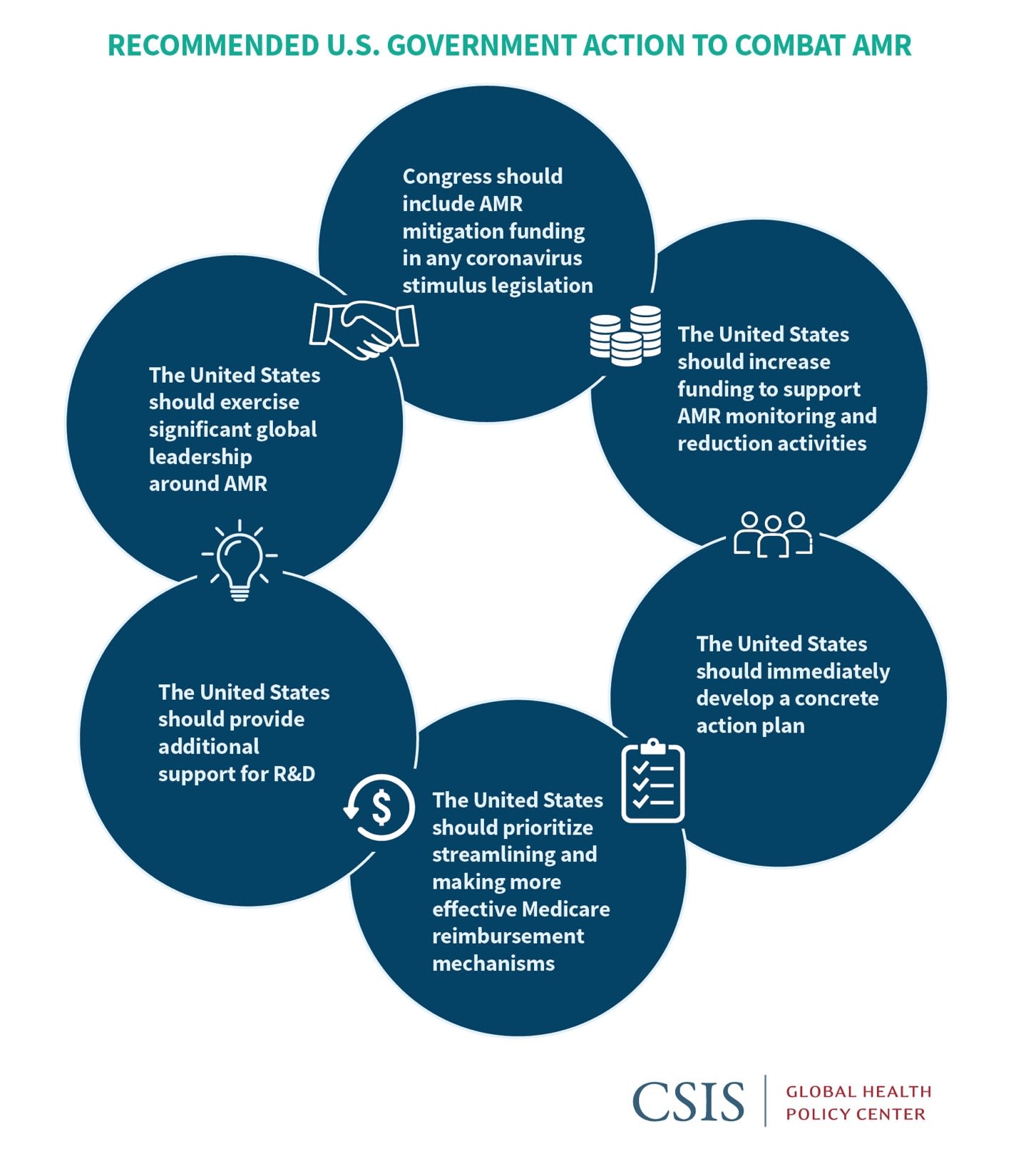
While the U.S. government has made substantial headway in supporting domestic and international AMR reduction policies, more resources and attention are crucial. To address the threat of AMR, we suggest the following actions:
- Congress should include AMR mitigation funding in any coronavirus stimulus legislation to support research, diagnostics, and countermeasure development and to enhance Medicare payments for antibiotic treatments.
- The United States should increase funding to support AMR monitoring and reduction activities, including those related to health facility policies, laboratory support, and surveillance across the human, animal, and environmental spectrum (the One Health approach), based on recommendations from the Centers for Disease Control and Prevention (CDC).
- The United States should immediately develop a concrete action plan that is agreed to and supported by industry to create effective incentives for the development and market sustainability of new antimicrobials and other countermeasures.
- The United States should prioritize streamlining and making more effective Medicare reimbursement mechanisms and other incentives that confer appropriate social and long-term medical value to AMR- mitigating innovations.
- The United States should provide additional support for R&D related to microbial diagnostics and select vaccines.
- The United States should exercise significant global leadership around AMR, including advancing pooled international resources for antibiotic research and development and enhanced surveillance, stewardship, and containment activities.
A PROLIFERATION OF DANGEROUS BUGS
Starting with the discovery of penicillin in 1928, medical researchers developed the capacity to treat the dangerous infections that have plagued humankind for millennia. For two decades, starting in the 1950s, the creation of a range of new antimicrobials proved lifesaving for many.1 But increased use and misuse of antimicrobials over the years has allowed some pathogens to withstand available treatments, thus prolonging and intensifying human infections. Multiple factors lead to antimicrobial resistance: overuse (i.e., prescribing antibiotics for conditions such as viral infections, on which the drugs have no effect); inappropriate use (i.e., not taking the right drug at the right time for the full duration, allowing some pathogens to survive treatment); excessively broad reliance in food animals; presence of antibiotics in wastewater where the drugs accumulate from human and animal waste; and, in some countries, drug manufacturing runoff.2 Another driver of AMR is the increased availability of antimicrobials in developing countries with inadequate control mechanisms.
Resistant infections require more and sometimes stronger drugs, which may be costly and have severe side effects. Pathogens for which no or few antimicrobials are effective are called “pan-resistant” and can result in patient death. AMR increases overall health care costs, as severely afflicted patients often are hospitalized, some for lengthy periods. While many resistant infections occur in hospitals and other care facilities, they also are found in the community, spread through unsafe sex practices and contact with contaminated food and water.
AMR has received increasing attention in the last several decades as more cases are recorded both in the United States and around the world, but the full extent of the problem in less-developed countries remains unknown in the absence of widespread and effective monitoring and surveillance. AMR is truly a global threat, as people, animals, and goods carry infections across borders with them.
MITIGATING AMR
Mitigating AMR requires a multi-pronged approach that involves agricultural practices, health facility policies, payment reform, and continuous research and development. Since microbes begin to develop resistance as soon as a new drug is available, stemming AMR requires preventing infections, implementing effective stewardship programs, and developing new treatments. AMR reduction requires educational programs for both the public and health care providers to ensure appropriate use of available treatments; the implementation of rigorous infection control mechanisms in health care facilities; improved hygiene with clean water for hand washing and safe food preparation; and the establishment of antimicrobial stewardship programs that ensure treatments are used correctly. Beyond human drug use, stemming AMR requires close monitoring and appropriate antibiotic use in food and crop production, including animal husbandry and crop management policies that decrease occurrence of infection and reduce the need for antimicrobials. AMR is truly a global threat, as people, animals, and goods carry infections across borders with them.
While public health officials have made significant progress in identifying AMR as a serious threat and some progress in addressing the issue, largely in hospital settings, prevention strategies are still lacking and need to be combined with surveillance and containment approaches. Further, urgent action is needed to ensure a pipeline of new antimicrobials that is stable and robust enough to meet health care delivery needs. In fact, only two new classes of antibiotics have been created since the 1970s, and most large drug companies have stopped work in the area, citing insufficient return on investment.3 New drug development is expensive and time-consuming, requiring years of effort and up to $2.6 billion.4 While potentially lifesaving, antimicrobials should be used sparingly and are necessary only in a small population, producing an unacceptable rate of return for many large pharmaceutical manufacturers. Some smaller companies have continued to develop drugs but find they often do not have sufficient sales and marketing infrastructure to ensure market sustainability. Alarmingly, several smaller drug manufacturers that developed successful drugs have recently gone bankrupt, focusing attention on the dire state of the antimicrobial market and highlighting the need for additional resources and creative solutions not only for antibiotic development but also for AMR prevention and treatment alternatives.5
While the fragile market is the most visible and vexing manifestation of AMR, resources and innovations are essential for other mitigation strategies as well. In particular, mechanisms are needed to facilitate development of diagnostics that can quickly identify potentially resistant microbes. Matching a microbe with the most effective, narrowest spectrum therapy available not only benefits patients but also makes the best use of existing drug arsenals, can reduce side effects and the potential for further resistance, and can keep the infection from spreading.
THE U.S. GOVERNMENT AND AMR
The U.S. government redoubled its efforts to mitigate AMR over the last decade, increasing monitoring and prevention activities, supporting research and development, and revising how Medicare reimburses for new antibiotics.6 A 2013 CDC report highlighting AMR and the “potentially catastrophic consequences of inaction” led President Obama to direct the National Security Council and the Office of Science and Technology Policy to develop a plan to address the threat.7 The result was a National Strategy for Combating Antibiotic-Resistant Bacteria issued in September 2014.8 The strategy outlined five goals:
- Slow the development of resistant bacteria and prevent the spread of resistant infections.
- Strengthen national surveillance efforts in both human and animal settings to combat resistance.
- Advance development and use of rapid and innovative diagnostic tests for identification and characterization of resistant bacteria.
- Accelerate basic and applied research and development for new antibiotics, other therapeutics, and vaccines.
- Improve international collaboration and capacities for antibiotic resistance prevention, surveillance, control, and research and development.
To move further on the issue, Obama also issued an executive order creating the Task Force for Combating Antibiotic-Resistant Bacteria.9 Made up of multiple federal departments and agencies, the panel was charged with developing a plan to actualize the strategy’s goals. The National Action Plan for Combating Antibiotic Resistance was released in March 2015 and called for:
- Prudent use of antibiotics in both health care and agricultural settings, known as antibiotic The goal is to make the most effective use of existing antibiotics—providing “the right antibiotic at the right time at the right dose for the right duration”—to both reduce resistance and prolong the effective lifespan of drugs.
- Employing a “One Health” approach that combines monitoring of human and animal pathogens. Activities include creating a regional public health laboratory network to standardize testing and genetic characterization of bacteria. These activities would be coupled with increased monitoring of antibiotic sales, use, resistance, and management practices at multiple points along the food production chain.
- Improving diagnostic tests for quicker, more accurate identification of resistant bacteria.
- Fostering public/private partnerships to support basic and applied research, clinical trial networks, and attractive investment opportunities for new drugs and vaccines.
- Improving international collaboration to foster global prevention, surveillance, and control capabilities and encourage new drug research and development.10
Instituting the national action plan involves a variety of federal agencies and departments overseeing health facility policies and payments; laboratory standards and operation; drug, vaccine, and diagnostic development; and agricultural practices. A Year 3 report on the plan shows considerable progress. Among highlights are increased antibiotic stewardship at long-term care facilities and hospitals; development of new point-of-care diagnostics; continuous monitoring of resistance in the U.S. public and military populations; federal support for increased laboratory capacity in all 50 states, five large cities, and Puerto Rico, including regional labs; increased funding for prevention programs nationwide; and wider access to critical data and resources to spur innovations in diagnostics and drug development.11

ENSURING EFFECTIVE INFECTION CONTROL
Despite substantial progress, more work is ahead. A recent report from the CDC concluded that while AMR-related deaths have decreased since its first report in 2013, the number and scope of AMR threats in the United States is greater than initially understood. “This suggests that U.S. efforts—preventing infections, stopping the spread of bacteria and fungi, and improving use of antibiotics in humans, animals, and the environment—are working, especially in hospitals,” the report said.12 But with more than 2.8 million resistant infections and 35,000 deaths a year, the number of people afflicted is still unacceptably high.13 The CDC lists 18 specific AMR threats in three categories—urgent, serious, and concerning—and also added a “watch list” of potentially problematic pathogens.14
The CDC urged greater resources and attention to enhance AMR prevention and control, including creating better detection and monitoring tools and data, an expanded detection and response workforce, and increased collaboration between public health and health care providers. It also called for new antibiotics and diagnostics, better strategies for enhancing antibiotic stewardship, and better understanding of AMR in the environment and its impact on public health.
ENCOURAGING SUSTAINABLE ANTIMICROBIAL DEVELOPMENT
In addition to preventing infections, stemming AMR also requires development of new drugs and alternative treatments. Especially since bacteria and other pathogens begin to develop resistance to new drugs as soon as they are introduced, there will always be a need for new remedies. But fostering and sustaining a pipeline of new antimicrobials has proven to be a daunting challenge given the difficulty and expense of developing new drugs and the lack of a clear return on investment.
The U.S. government has instituted several programs and practices to aid the development of new drugs. One approach is the establishment of “push incentives,” which provide funding directly to drug companies to develop new products. The U.S. Department of Health and Human Services offers funding directly to antibiotics manufacturers through the Biomedical Advanced Research and Development Authority (BARDA). Established in 2006, BARDA aids the transition of medical countermeasures from research through development, FDA consideration, and inclusion in the Strategic National Stockpile.15 In 2011, BARDA developed a branch to support antimicrobial development. Since then, BARDA has provide more than $1.2 billion to aid the development of 70 new antimicrobial candidates. Of the 54 antibacterial products BARDA is currently supporting, 8 have progressed into Phase 3 clinical development, and an additional 3 have been approved by the FDA.16
Fostering and sustaining a pipeline of new antimicrobials has proven to be a daunting challenge given the difficulty and expense of developing new drugs and the lack of a clear return on investment.
But the recent failure of companies that had effective, approved products has government officials searching for new ways to assist. While providing development money will help revitalize the antibiotic pipeline, the drugs’ unique profitability and marketing challenges indicate the need for additional approaches. “BARDA simply cannot continue to provide non-dilutive investment, only to have companies collapse and their newly minted antibiotics shelved or lost completely,” said former BARDA Director Rick Bright.17
Most importantly, new reimbursement methodologies need to be developed for antibiotics, including through Medicare. The majority of both antibiotic resistant infections and deaths occur among Medicare beneficiaries, and Medicare payment policies traditionally set the stage for other payers. While the Centers for Medicare and Medicaid Services (CMS) has increased Medicare payments for some antibiotics, hospital officials say the payment mechanism is cumbersome and has done little to create a stronger market for the drugs.18 The CMS is considering how best to proceed through its current regulatory authorities.19 Ultimately, efforts need to be made to recognize the societal and overall medical value of antibiotics beyond the direct benefit to the patient being treated. Without effective antibiotics, commonly performed medical procedures can become life threatening, a situation that would have dire consequences for both human health advances and medical economics. Determining the correct price for antibiotics based on their true societal value would require tapping into the expert opinion of health economists, policy experts, ethicists, and others who could develop a value framework that reflects the costs of not having effective drugs available.
While officials work through the larger reimbursement conundrum, some shorter-term options are being considered. In addition to CMS attention to the issue, other options include expanding bulk government purchases of antibiotics, helping smaller companies connect with larger manufacturers to tap into their marketing and distribution networks, and developing a standing clinical trial network to provide more streamlined testing of new drugs. BARDA made its first steps into large-scale procurement of antibiotics with a 5-year $169 million award to Paratek Pharmaceuticals on December 19, 2019. If the FDA approves the drug, Nuzyra, a treatment for anthrax lung infections, BARDA will work with the manufacturer to purchase a supply.20 This is a clear example of how the U.S. government can leverage procurements as a pull mechanism to support commercial-stage antimicrobial companies. While some of the options being considered would require new authorities for responsible agencies, all of them would require additional funding from Congress.
Legislators are looking at other options as well. A bipartisan proposal introduced last year, the Developing an Innovative Strategy for Antimicrobial Resistant Microorganisms (DISARM) bill, would create Medicare add-on payments for hospitals that use a qualifying antibiotic to treat a life-threatening infection.21 However, although it has bipartisan support, the bill is languishing, caught up in the political optics of granting more funding to pharmaceutical companies when concern over drug expenses remains high.
There are other payment options being developed as well. One proposal is a subscription model that would provide access to antibiotics for a flat and predictable recurring payment. The model would guarantee a revenue stream for a product regardless of volume of use and would reflect health value beyond those provided to the patient actually using the drug. Further, since it de-links payments from drug use volume, it would eliminate the need for companies to drive uptake in order to make a profit, thus comporting to principles of antibiotic stewardship.22 The United Kingdom’s National Health Service is piloting a subscription model whereby it will pay pharmaceutical companies in advance for access to antibiotics rather than paying based on volume.23 Another bulk purchase pilot is being launched in Sweden.24 Other options to explore include advance commitment mechanisms such as that outlined by the Center for Global Development for Tuberculosis.25
RESISTANT INFECTIONS KNOW NO BORDERS
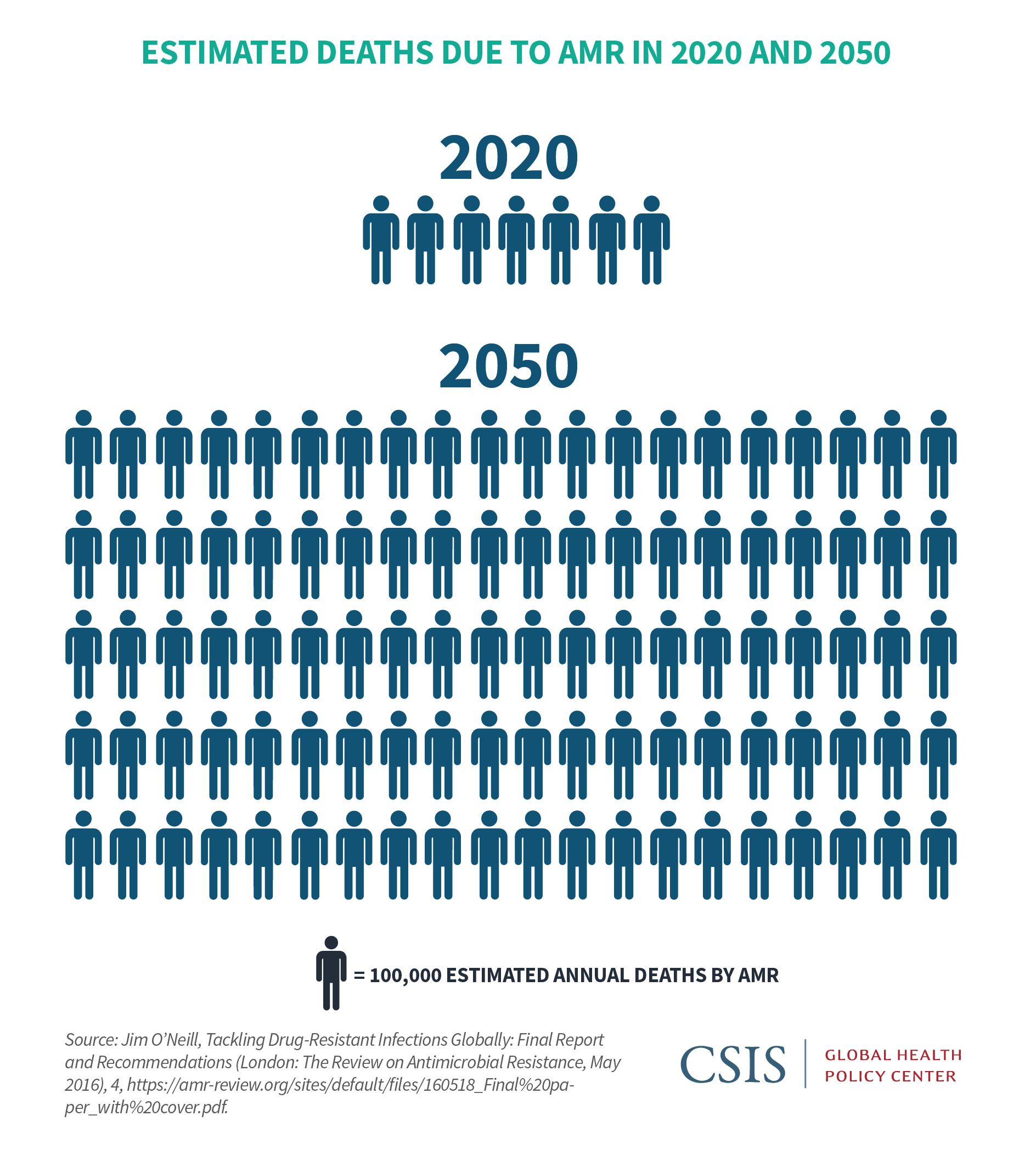
While AMR is a pressing issue in the United States, its implications are even more dire globally. In fact, the World Health Organization (WHO) lists AMR among its top threats to global health.26 While monitoring of AMR- related morbidity and mortality is sorely lacking in all but the most advanced economies, estimates attribute 700,000 deaths globally per year to resistant infections. By 2050, 10 million lives a year and $100 trillion of economic output could be at risk.27 The international community has held high-level meetings on the issue and developed recommendations and reports. Consensus has evolved that top actions include ensuring appropriate use of antibiotics both in humans and agriculture; eliminating untreated effluent in both animal and human health; bolstering infection prevention and control; and ensuring access to appropriate antibiotics to all who need them.28 Efforts have been made toward those goals over the last decade, including the creation of the Transatlantic Taskforce on Antimicrobial Resistance in 2009; the Global AMR Research and Development Hub launched out of the G20 in May 2018; and the Interagency Coordination Group on Antimicrobial Resistance report of April 2019.29
Despite increased efforts, even determining the scope of the problem globally remains a challenge. The WHO’s new Global Antimicrobial Surveillance System (GLASS) documented antibiotic resistance in more than 500,000 people in 22 countries, although only one quarter of the United Nations’ 194 member states have agreed to participate in the network.30 Greater attention is urgently needed to strengthen national and global AMR monitoring and mitigation capabilities, increase global efforts toward antibiotic development, and create a global governance mechanism, such as the proposed High- Level AMR Commission and Global Steering Board outlined by the United Kingdom’s Dame Sally Davies and others.31 Greater global effort on AMR mitigation is a top priority for the CDC, which urges better infection control worldwide; broader use of vaccines that can decrease antibiotic use; improved use and access to antibiotics in all countries; universal access to clean water and effective sanitation; and expanded global laboratory capacity to detect AMR threats.32 As part of its effort to increase attention to the issue, the CDC launched a global AMR Challenge at the United Nations in September 2018 to encourage governments, businesses, and non- governmental organizations worldwide to make formal commitments to AMR reduction.33
AMR REQUIRES IMMEDIATE SUSTAINED U.S. LEADERSHIP AT HOME AND ABROAD
In community clinics, doctor’s offices, and hospitals the world over, AMR threatens health and health care provision. In the United States alone, it is responsible for more than 35,000 preventable deaths every year. The United States has made significant strides in addressing the issue, but more attention and funding are needed. Increased antibiotic use and deaths from secondary infections related to coronavirus need to be highlighted to spur more action on AMR. Secondary bacterial infections killed as many as half of patients in study populations during the 2009 H1N1 influenza pandemic and are a significant concern with Covid-19 as well.34 “The challenge of antibiotic resistance could become an enormous force of additional sickness and death across our health systems as the tool of coronavirus pneumonia stretches critical care units beyond their capacity,” according to former CDC Director Julie Gerberding.35 Recognizing the threat, some lawmakers initially considered funding for antibiotic research and Medicare payment enhancements for inclusion in recent coronavirus stimulus bills, but none of the provisions were enacted.
Particularly in light of the coronavirus crisis and the resulting likelihood of increased and devastating secondary infections over the next months, rapid and intense attention is needed to help stem AMR. For the longer term, global AMR surveillance and monitoring need to ramp up immediately to give a more complete understanding of the threat. At the global level, high- level action and leadership is paramount. The U.S. government, in addition to improving infection control and antibiotic stewardship at home, should play a lead role in developing mechanisms to sustain antibiotic development and spur creation of innovative alternatives. Further, as a public health leader globally, the United States should actively formulate, support, and advance global efforts to reduce AMR to help protect Americans at home and abroad.

Nellie Bristol is a senior associate with the Global Health Policy Center at the Center for Strategic and International Studies in Washington, D.C.
This brief is a product of the CSIS Commission on Strengthening America’s Health Security, generously supported by the Bill & Melinda Gates Foundation.
The author would like to thank the following individuals for their contributions to this report:
Mark Albrecht, Chief, Antibacterial Program, Division of CBRN Medical Countermeasures, U.S. Biomedical Advanced Research and Development Authority
Luciana Borio, Vice President, In-Q-Tel
Michael Craig, Antibiotic Resistance Coordination and Strategy Unit, U.S. Centers for Disease Control and Prevention
Sarah Despres, Director, Government Relations, The Pew Charitable Trust
Philip Ferro, Director for Countering Biological Threats, White House National Security Council
Lauren Fabina, White House National Security Council
Keiji Fukuda, Professor, University of Hong Kong
Andi Fristedt, Deputy Health Policy Director, Senator Patty Murray
Christopher Houchens, Acting Director, Division of CBRN Medical Countermeasures, U.S. Biomedical Advanced Research and Development Authority
Sarah Jones, Partnerships, Antibiotic Resistance Coordination and Strategy Unit, U.S. Centers for Disease Control and Prevention
Mary Lancaster, International Policy Officer, Defense Threat Reduction Agency, U.S. Department of Defense
Nicole Lurie, Distinguished Health Policy Fellow, Leonard Davis Institute of Health Economics, University of Pennsylvania
Christine Sizemore, Director of International Relations, Fogarty International Center, National Institutes of Health
Padmini Srikantiah, Senior Program Officer, the Bill &Melinda Gates Foundation
Kathy Talkington, Director, Antibiotic Resistance Project, the Pew Charitable Trust
Paige Waterman, Assistant Director for Biological Threat Defense, White House Office of Science and Technology Policy
Anne Yu, AMR Division, World Health Organization
CSIS Briefs are produced by the Center for Strategic and International Studies (CSIS), a private, tax-exempt institution focusing on international public policy issues. Its research is nonpartisan and nonproprietary. CSIS does not take specific policy positions. Accordingly, all views, positions, and conclusions expressed in this publication should be understood to be solely those of the author(s).
© 2020 by the Center for Strategic and International Studies. All rights reserved.
Please consult the PDF for references.